Computer System Validation (CSV) is an essential process that ensures that computer systems and software perform the way they are intended to work.
Also Read
CSV is crucial for regulated companies that operate in industries like pharmaceuticals, biotechnology, and medical devices.
These companies must comply with regulations and guidelines set by regulatory bodies like the FDA, EMA, and others.

The process of computer system validation involves testing, validating, and qualifying a regulated computerized system to ensure that it performs consistently and reliably.
The process is critical to ensuring that electronic records and signatures are trustworthy, reliable, and accurate.
The validation process can take many forms, depending on whether it is a new implementation or an upgrade to an existing system.
In today’s highly regulated business environment, computer system validation is a crucial aspect of ensuring that companies comply with regulatory requirements.
It is essential to ensure that computer systems and software perform as intended, providing accurate and reliable data.
As such, companies must take the necessary steps to validate their computer systems and software to ensure that they meet regulatory requirements and operate effectively.
Understanding Computer System Validation
Computer System Validation (CSV) is an essential process that regulated companies must undertake to ensure that their software or system is functioning correctly and as intended.
This section will delve into the key components of CSV and why it is so important.

Importance of CSV
CSV is critical for regulated companies to ensure that their systems are operating correctly and not performing in unintended ways.
It is necessary to ensure that the software or system meets the required specifications and performs as expected.
This is especially important in regulated industries such as pharmaceuticals, where a mistake in software can have severe consequences.
CSV is also necessary to meet regulatory requirements. Regulators such as the FDA require validation of computer systems to ensure that the data produced by the system is reliable, accurate, and consistent.
Failure to comply with these regulations can result in fines, product recalls, and damage to a company’s reputation. The “V Diagram” was popularized by industry organizations such as ISPE via GAMP Guides.
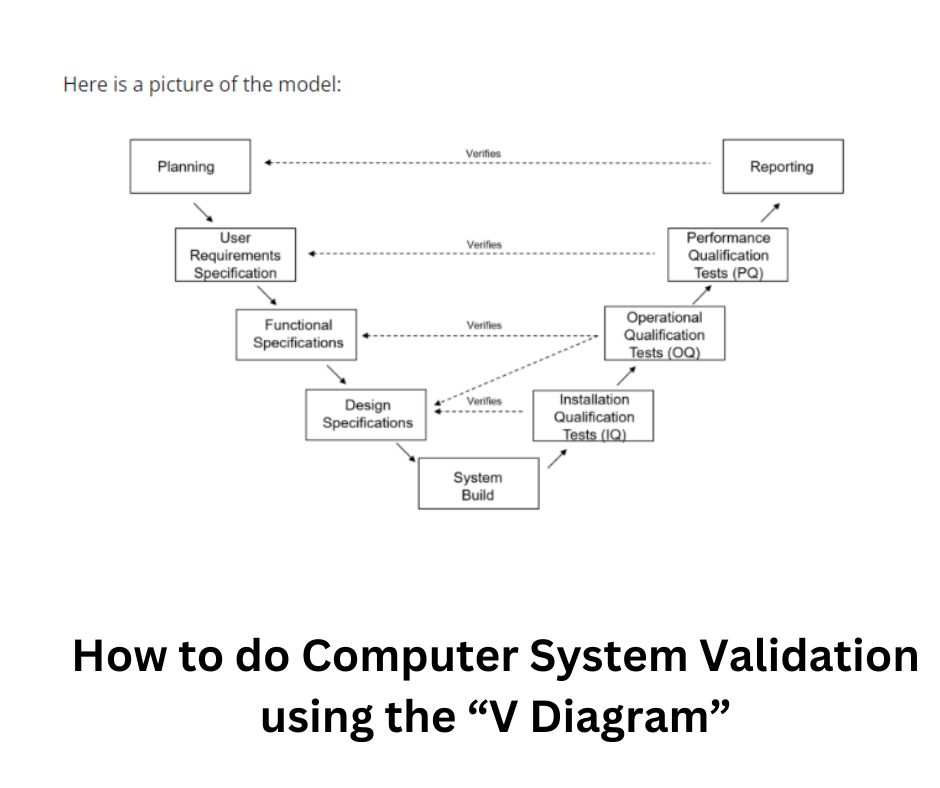
Key Components of CSV
There are several key components of CSV, including:
- Planning: This involves defining the scope, objectives, and strategy of the validation project. It also involves identifying the risks and determining the resources required for the project.
- User Requirements: This involves defining the requirements of the system from the user’s perspective. It includes the functional and non-functional requirements of the system.
- Design: This involves developing a design specification that meets the user requirements. It includes the hardware and software components, as well as the interfaces between them.
- Testing: This involves testing the system to ensure that it meets the user requirements and design specification. It includes unit testing, integration testing, and system testing.
- Documentation: This involves documenting the entire validation process, including the planning, user requirements, design, testing, and results.
Overall, CSV is an essential process for regulated companies to ensure that their systems are functioning correctly and meeting regulatory requirements.
By following the key components of CSV, companies can ensure that their systems are reliable, accurate, and consistent.
Regulatory Framework

Computer System Validation (CSV) is a process that ensures computerized systems are operating in a consistent and reproducible way, complying with regulatory rules.
The regulatory framework for CSV is provided by various regulatory authorities, including the US Food and Drug Administration (FDA) and international standards organizations.
FDA’s Role
The FDA’s role in CSV is to ensure that computerized systems used in regulated industries, such as pharmaceuticals, biotechnology, and medical devices, are validated and meet regulatory requirements.
The FDA enforces regulations, such as 21 CFR Part 11, which mandates electronic records and signatures to be trustworthy, reliable, and equivalent to paper records.
The FDA provides guidance documents, such as the “General Principles of Software Validation,” to help companies understand the validation process and how to comply with regulatory requirements.
The FDA also conducts inspections to ensure that companies are following the regulations and guidelines.
International Standards
International standards organizations, such as the International Organization for Standardization (ISO), provide guidance on CSV.
ISO 13485:2016, for example, provides requirements for the validation of software used in the quality management system for medical devices.
ISO/IEC 12207:2017 provides a framework for the development, operation, and maintenance of software.
Other international standards, such as the Good Automated Manufacturing Practice (GAMP) guidelines, provide a risk-based approach to CSV.
The GAMP guidelines provide a framework for the validation of computerized systems used in the pharmaceutical and biotechnology industries.
In summary, regulatory authorities, such as the FDA, and international standards organizations provide the regulatory framework for CSV.
Companies must comply with these regulations and guidelines to ensure that their computerized systems are validated and meet regulatory requirements.
CSV Process

Computer System Validation (CSV) is an essential process for regulated companies to ensure that their software or system is performing the way it is supposed to work.
The CSV process involves several steps that must be followed to prove that the system is working as intended. The following are the sub-sections of the CSV process:
Planning and Documentation
Before starting the CSV process, it is essential to develop a plan that outlines the scope of the validation project. The plan should include a description of the system, its intended use, and the validation approach.
Documentation is also critical in the planning stage, as it provides a record of the validation process and helps ensure that the system meets the regulatory requirements.
Risk Assessment
Risk assessment is an important step in the CSV process, as it helps identify potential risks associated with the system and enables the development of appropriate risk mitigation strategies.
The risk assessment process involves identifying hazards, analyzing the risks associated with those hazards, and developing strategies to mitigate those risks.
System Testing
System testing is a critical step in the CSV process, as it involves testing the system to ensure that it is functioning as intended. The testing process should include functional testing, performance testing, and security testing.
The results of the system testing should be documented and reviewed to ensure that the system meets the regulatory requirements.
System Maintenance
System maintenance is an ongoing process that involves monitoring the system to ensure that it continues to function as intended. This includes regular system backups, software updates, and security patches.
The maintenance process should be documented, and all changes to the system should be reviewed to ensure that they do not impact the system’s performance or compliance with regulatory requirements.
In conclusion, the CSV process is critical in ensuring that computer systems are functioning as intended and meeting regulatory requirements.
By following the steps outlined in the CSV process, companies can ensure that their systems are safe, reliable, and compliant with regulatory requirements.
Challenges and Solutions in CSV
Computer System Validation (CSV) is a necessary process for ensuring that computerized systems are performing as intended and meeting regulatory requirements.
However, there are several challenges that organizations face when implementing CSV. In this section, we will explore some common pitfalls and best practices to overcome these challenges.
Common Pitfalls
Lack of Understanding
One of the biggest challenges in CSV is a lack of understanding of the process. Many organizations do not understand the regulatory requirements for CSV, leading to incomplete or inadequate validation efforts. This can result in costly rework and delays in product launch.
Poor Documentation
Another common pitfall is poor documentation. CSV requires extensive documentation to ensure that all aspects of the system are validated and that the validation can be reproduced in the future.
Poor documentation practices can lead to incomplete or inadequate validation efforts, making it difficult to demonstrate compliance to regulatory agencies.
Inadequate Testing
Inadequate testing is another common pitfall in CSV. Organizations may not perform sufficient testing to ensure that the system is functioning as intended.
This can result in errors or defects that are not identified until the system is in use, leading to costly rework and delays in product launch.
Best Practices
Risk-Based Approach
A risk-based approach is a best practice for successful CSV. This approach involves identifying and assessing the risks associated with the system and focusing validation efforts on the areas of highest risk.
This approach ensures that validation efforts are focused on the most critical aspects of the system, reducing the risk of errors or defects.
Adequate Documentation
Adequate documentation is also a best practice for successful CSV. This includes detailed documentation of the validation plan, testing protocols, and results.
Documentation should be clear, concise, and easily reproducible to ensure that the validation can be demonstrated to regulatory agencies.
Comprehensive Testing
Comprehensive testing is another best practice for successful CSV. Organizations should perform thorough testing to ensure that the system is functioning as intended. This includes functional testing, performance testing, and security testing.
Comprehensive testing reduces the risk of errors or defects and ensures that the system is compliant with regulatory requirements.
Overall, successful CSV requires a thorough understanding of the regulatory requirements, comprehensive documentation, and adequate testing.
By following best practices and avoiding common pitfalls, organizations can ensure that their computerized systems are compliant and functioning as intended.
Future of CSV
Computer System Validation (CSV) is a crucial process in regulated industries such as pharmaceuticals and medical devices. As technology continues to evolve, the future of CSV is likely to see changes and advancements in the way it is performed.
One trend that is expected to shape the future of CSV is the increased use of automation. As more and more systems become automated, it will become necessary to validate these systems to ensure they are functioning as intended.
This will require new approaches to CSV that are specifically designed to address the unique challenges posed by automated systems.
Another trend that is likely to impact the future of CSV is the growing importance of data integrity. With the increasing reliance on data in regulated industries, it is essential to ensure that data is accurate, complete, and secure.
This will require new validation strategies that are focused on ensuring data integrity throughout the entire system lifecycle.
Finally, the future of CSV is likely to see an increased emphasis on risk-based validation. Rather than validating every aspect of a system, risk-based validation focuses on identifying the most critical components and validating those first.
This approach can help to streamline the validation process and reduce costs while still ensuring that critical components are validated appropriately.
Overall, the future of CSV is likely to see significant changes as technology continues to evolve and new challenges emerge.
However, by staying up-to-date with the latest trends and best practices, organizations can ensure that their CSV processes remain effective and efficient in the years to come.
Frequently Asked Questions
What are the guidelines for computer system validation?
Computer system validation (CSV) is a process that ensures the computer system is performing as expected, and it is essential to follow certain guidelines to ensure that the process is effective.
The Good Automated Manufacturing Practice (GAMP) guidelines are widely used for CSV and provide a framework for the validation process.
The GAMP guidelines are developed by the International Society for Pharmaceutical Engineering (ISPE) and are updated regularly to reflect the latest industry practices.
What is the importance of computer system validation in pharmaceuticals?
Pharmaceutical companies are required to comply with strict regulations, and computer system validation is an essential part of ensuring that the data generated by computer systems is accurate, reliable, and secure.
Computer system validation helps to ensure that the computer systems used in pharmaceutical manufacturing, testing, and distribution are working correctly and that the data generated is accurate and reliable.
What is the role of FDA in computer system validation?
The FDA is responsible for regulating the pharmaceutical industry in the United States, and computer system validation is an essential part of ensuring compliance with FDA regulations.
The FDA provides guidance on computer system validation, and pharmaceutical companies must follow these guidelines to ensure that their computer systems are compliant.
What is the purpose of computer system validation SOP?
The purpose of a computer system validation Standard Operating Procedure (SOP) is to provide a standardized approach to the validation process.
The SOP outlines the steps that must be followed to ensure that the computer system is validated correctly and that the data generated is accurate and reliable.
What are the benefits of computer system validation certification?
Computer system validation certification provides assurance to employers that an individual has the necessary skills and knowledge to perform computer system validation effectively.
Certification can also help individuals to demonstrate their expertise and improve their career prospects.
Can you provide an example of a computer system validation checklist?
A computer system validation checklist is a tool used to ensure that all aspects of the validation process have been completed.
The checklist may include items such as verifying that the system requirements have been met, ensuring that the system is secure, and testing the system to ensure that it is working correctly.
An example of a computer system validation checklist can be found here.












